Our Engineering Process

BRD Lucchini follows a structured development process that brings clarity and consistency to engineering projects. Our four-stage approach ensures projects progress logically from requirements gathering through to implementation, with appropriate documentation and verification at each stage.
This methodology draws on principles from ISO13485 quality systems but applies them pragmatically across both medical and industrial projects. The result is a balanced approach that manages risk effectively without unnecessary paperwork or bureaucracy.

Gate 2: Design Development
- Technical drawings and 3D models
- Design documentation and calculations
- Functional prototypes as required
Verification testing is conducted to confirm the design meets the specified inputs and requirements. This is followed by validation testing to ensure the overall solution fulfils its intended purpose under actual or simulated use conditions.
This gate also encompasses most of our risk management activities, with systematic assessment and mitigation of potential failure modes throughout the design process.
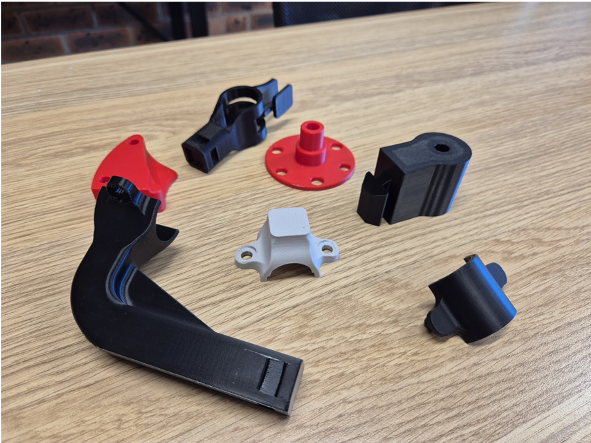

Gate 3: Market Readiness
Once the design is validated, Gate 3 focuses on preparing the product for production implementation. During this gate, we finalise the bill of materials (BOM), establish supply chain requirements, and complete all necessary technical files for manufacturing.
For medical devices, this gate may include regulatory submission preparation. For general engineering projects, documentation might focus on compliance with relevant standards and operational specifications.
Our involvement at this gate varies depending on the project scope and our role in the product lifecycle. Some clients require comprehensive management of the preparation phase, while others need focused technical support that complements their internal capabilities. Activities often include engagement with third-party certifiers, handover of production documentation, and finalisation of quality control procedures.

Gate 4: Implementation
The final gate covers the transition to production and field deployment. During Gate 4, we provide technical support during manufacturing setup or product installation, helping ensure the design intent is properly translated into the final product.
This gate includes gathering field feedback and conducting performance testing under actual operating conditions. Based on these results, we can implement refinements and optimisations if required to address any operational issues identified.
As with previous gates, our level of involvement at this stage adapts to suit the specific project requirements and client capabilities. For some projects, we provide comprehensive implementation management; for others, targeted technical assistance during critical phases of deployment.
Risk Management Through Transparency
Our gated development process incorporates systematic risk management methodologies such as FMEA (Failure Mode and Effects Analysis) to identify potential issues before they impact project outcomes.
This structured approach has demonstrably reduced redesign cycles, shortened time-to-market, and provided auditable justification for critical engineering decisions.
We apply this risk management proactively at each development stage. For mining equipment, this might mean identifying vibration-induced failures during design rather than field testing. For medical devices, it means addressing usability risks before verification testing rather than during regulatory review.
Our approach is built on three core elements: rigorous testing protocols to validate design assumptions, comprehensive documentation that creates traceability from requirements through to implementation, and structured client communication that ensures technical and business requirements remain aligned throughout development.
This methodology has proven particularly valuable for projects where failure costs are high – whether through operational downtime, regulatory delays, or market reputation.


